Armbrust American Independently Verified Lab Results

Below are independently verified lab results from the several required tests, in which Armbrust masks returned better margins than required by established standards.
FDA Letter: 510K Approval - Armbrust Surgical Masks
Disposable Face Mask Colors Lab Results (Nelson Labs)
Better Mask High Filtration System Lab Results:
- Armbrust Surgical Masks (Better Mask Kit) Test Results (PDF)
- FTM Essential Brace (Better Mask Kit) Test Results (PDF)
Filtering Facemask Respirator (AA-1776) Lab Results:
Surgical Mask Lab Results:
BFE Test ( BFE Test Full Report)
“Bacterial Filtration Efficiency” is a test that shows the amount of bacteria that can get through our masks. Nelson Labs used aerosolized Staphylococcus Aureus to test Armbrust masks, and despite accidentally using nearly 10 times the amount that is usually required for a test like this, they passed with 99.2%+ Efficiency -- well above the ASTM II & III requirements.

PFE Test ( PFE Test Full Report)
“Particle Filtration Efficiency” tests the size of particles that can pass through a mask. Armbrust masks were well above the 98% required for ASTM II. It should be noted that a PFE rating of 98% typically blocks most particles down to about 0.3 microns — the Staphylococcus Aureus bacteria used in our BFE test can get as small as 0.1 micron (about the size of the CoronaVirus).

Differential Pressure Test ( Differential Pressure Test Full Report)
This test is used to determine the breathability of Armbrust surgical masks. Sure, you might be able to block viruses, but can you still breath? Even with our higher BFE of 99%+, we passed with much better breathability than normal — in most cases under 4.9 (mm H2O/cm2) versus the requirement of being less than 6.0 (mm H2O/cm2) — also attached.

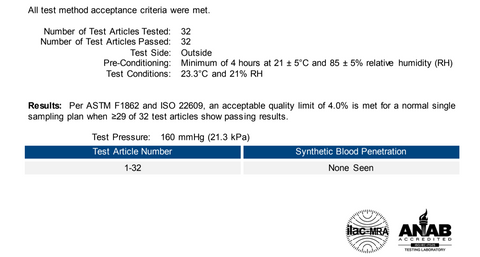
Synthetic Blood Penetration Test ( Synthetic Blood Penetration Test Full Report)
This procedure was performed to evaluate surgical face masks and other types of protective clothing materials designed to protect against fluid penetration. The purpose of this procedure is to simulate an arterial spray and evaluate the effectiveness of the test article in protecting the user from possible exposure to blood and other body fluids. All 32 masks tested passed synthetic blood spray at 160 mmHg with no penetration seen. ( See the test in action)
Flammability Test ( Flammability Test Full Report)
Meant to ensure that a face mask will not ignite and burn for more than 3.5 seconds. All of our masks burned for less than one second, passing this requirement.

Cytotoxicity Test ( Cytotoxicity Test Full Report)
Designed to test if people will have a reaction when wearing our masks. On a scale of 0–4, with zero being no reaction and four being a severe reaction, masks that on average hit a 2 or lower pass this test. All of our masks received a grade of 0 and passed.

Irritation Intracutaneous Toxicity Test ( Irritation Intracutaneous Toxicity Test Full Report)
"The purpose of the study is to determine the potential irritation effects of the test article extract as a result of an intracutaneous injection in New Zealand White rabbits."
Sensitization Kligman Maximization Test ( Sensitization Kligman Maximization Test Full Report)
"The purpose of the study was to determine the potential allergenic or sensitizing capacity of the test article. The study was used as a procedure for screening of contact allergens in guinea pigs and extrapolating the results to humans, but it does not establish the actual risk of sensitization."